Three Advocate Health Care hospitals were recognized as high-performing clinical locations in a research study that led to the recent U.S. Food and Drug Administration (FDA) approval of a new device to treat stroke.
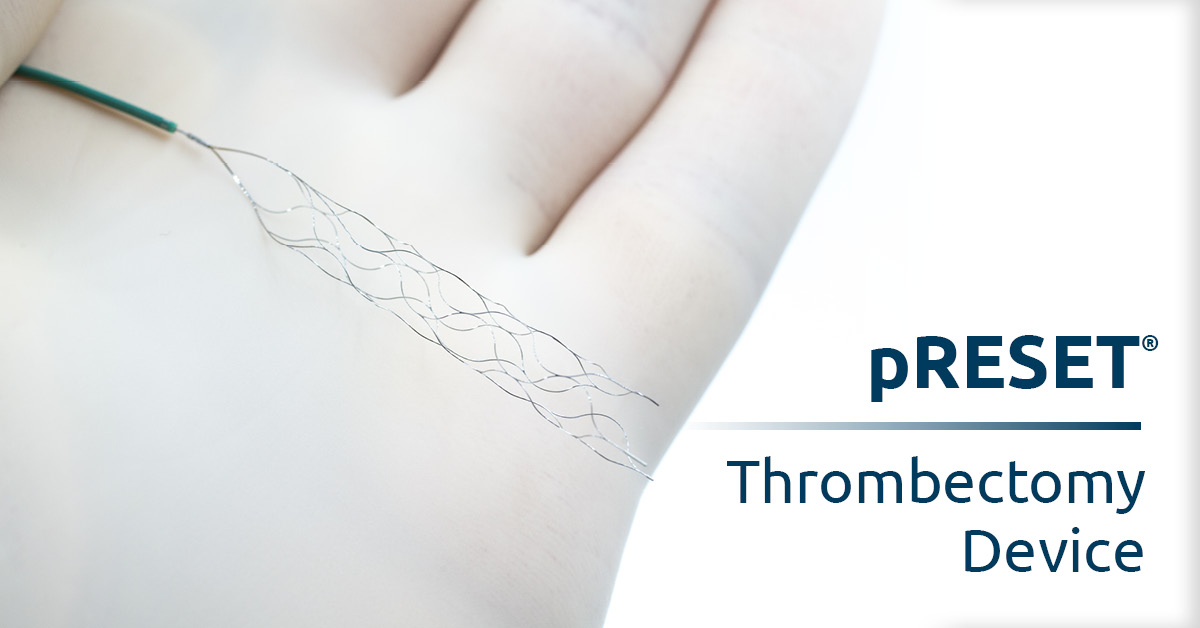
The pRESET® Thrombectomy Device received FDA clearance for use to treat acute ischemic stroke in the U.S. based on results from the “pRESET for occlusive stroke treatment (PROST)” clinical trial.
Nearly 800,000 people in the U.S. suffer from a stroke each year – that’s one person every 40 seconds – and almost 90% are caused by blood vessel blockages, according to the U.S. Centers for Disease Control and Prevention. Strokes are the number one cause of long-term disability and the fifth leading cause of death.
“The pRESET device is designed to quickly remove a blockage-causing thrombus, or blood clot, and restore blood flow to the brain,” said neurosurgeon Demetrius Lopes, MD, Director of the Cerebrovascular Program, Surgical Director of the Advocate Health Care Stroke Network, and the study’s principal investigator for all three hospitals. “With stroke care, speed is important because time is brain. A lack of blood flow results in brain tissue death, which can lead to significant disability and loss of life.”
Advocate Lutheran General Hospital in Park Ridge, Illinois, Advocate Christ Medical Center in Oak Lawn, Illinois, and Advocate Illinois Masonic Medical Center in Chicago were together named the No. 2 clinical site based on timeliness of study launch, quality of data and total enrollment. Researchers at the three comprehensive stroke centers combined to enroll 23 study participants. The research team was recognized during the American Heart Association’s 2023 International Stroke Conference for excellence conducting a prospective randomized trial in stroke care.
“What’s unique about Dr. Lopes and our stroke team are their integration with our clinical research team,” said Melissa Kadar, Director of Advocate Aurora Research Institute’s Center of Excellence in Neuroscience Research. “By integrating the Research Institute’s informed consent process into our 24/7 stroke care and utilizing e-signature technology, we are able to prescreen patients who are experiencing stroke symptoms. That way, if they arrive at one of our comprehensive stroke centers in need of urgent and immediate stroke treatment, they have the option to join a research study without delaying their care.”
The pRESET device is inserted through a small incision in the leg via a very thin tube called a catheter that is visible by X-ray. A neurosurgeon inserts and threads the tube through the blood vessels in the brain. Once the clot is reached, the surgeon releases a compressed metal net from inside the tube, expands the net so it surrounds the clot, then retracts the net and clot back into the tube, and finally withdraws the tube from the blood vessels.
The PROST clinical trial directly compared the pRESET device to an existing mechanical thrombectomy device for the treatment of acute ischemic stroke.
“During the last 5 to 10 years, care for people with occlusive stroke has advanced incredibly due to smaller and better devices,” Dr. Lopes said. “Research and technology have brought us to a point where we now have multiple stroke treatment devices available to provide the best possible health outcomes for our patients.”
Researchers in the U.S. and Germany enrolled more than 300 total participants in the clinical trial.
To learn more about our research, visit aah.org/research.