Aurora St. Luke’s Medical Center in Milwaukee is the first site in Wisconsin to join an early feasibility clinical trial evaluating an investigational device designed to mimic the function of healthy blood vessels in people with pulmonary arterial hypertension, or PAH.
“The pulmonary arteries carry blood from the right side of the heart to the lungs,” said cardiologist Eric Roberts, MD, Advocate Aurora Research Institute’s principal investigator for the study. “Normally, the lung’s blood vessels easily expand and contract with the heart’s rhythm, which helps maintain consistent blood pressure and push blood onward to the lung’s capillaries for oxygenation. In patients with PAH, the lung’s blood vessels become narrow and stiff, increasing blood pressure in the lungs and forcing the heart to pump harder.”
The study, known as ASPIRE PH, will evaluate the safety and performance of the Aria CVTM Pulmonary Hypertension System in participants with PAH. It is believed to be one of the first device-only studies for PAH. (Previous studies have evaluated devices that deliver a medication to treat PAH.)
“If the Aria CV device performs as intended, participants may potentially see a reduction in symptoms caused by PAH, an improvement in overall quality of life and perhaps even prolonged life expectancy,” Dr. Roberts said.
Pulmonary arterial hypertension is a rare, progressive disorder, with between 500 and 1,000 new cases diagnosed each year in the U.S., according to the American Lung Association. Symptoms include shortness of breath, fatigue, swelling, dizziness, fainting, chest pain and heart palpitations. Clinicians often treat PAH with medications, but, for many, those medications may not work or may stop working. And, over time, PAH can overwork the heart, leading to heart failure.
This clinical trial will enroll participants at up to 10 sites across the country who remain symptomatic despite receiving at least two pulmonary hypertension medications for at least 90 days.
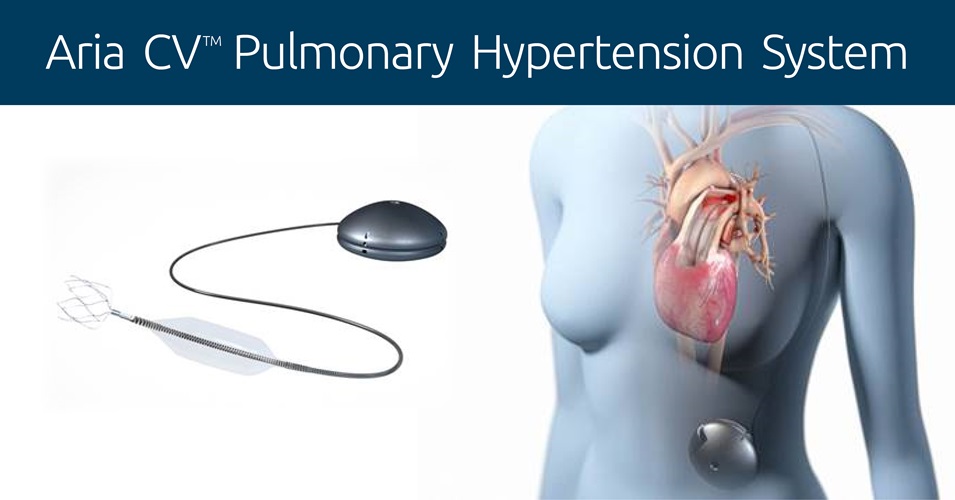
The Aria CVTM Pulmonary Hypertension System is designed to even out the blood pressure changes in the pulmonary blood vessels of people with PAH by replicating the normal function of a healthy, flexible pulmonary artery and assisting blood flow to the lungs. It does this by automatically inflating or deflating a balloon, implanted in the main pulmonary artery, when the pressure in the lung’s blood vessels changes.
When the heart contracts, pumping blood through the main pulmonary artery to the lungs, blood pressure in the artery rises because of the rigidity of the blood vessel due to PAH. To counteract this, the balloon deflates to even out the pressure. When the heart relaxes, blood pressure in the artery decreases, again because of the inflexibility of the blood vessel, so the balloon inflates, raising the blood pressure and pushing blood onward to the lungs.
“Although PAH is relatively rare, finding new treatments that can slow its progression is critical because no cure exists and the median survival for people with PAH is only seven years,” said Amit Acharya, PhD, chief research officer and system vice president for Advocate Aurora Health and the Research Institute. “Early feasibility studies such as this are an important first step in discovering innovative interventional treatments.”
ASPIRE PH is sponsored by Aria CV, IncTM, manufacturer of the investigational device.
Investigational Device. Limited by Federal (or United States) law to investigational use.
To learn more about Advocate Aurora’s research, visit aurora.org/research.