Aurora St. Luke’s Medical Center in Milwaukee is the first site in Wisconsin and only the fifth site in the country to join a research study evaluating a new investigational replacement heart valve.
This clinical trial, known as TWIST, assesses the safety and feasibility of a new transcatheter mitral valve replacement (TMVR) system designed to treat moderate to severe mitral regurgitation in people for whom open heart surgery is considered too risky.
“In people with mitral regurgitation, the leaflets of the mitral valve fail to close properly, which allows blood to leak backward in the heart, potentially leading to heart failure,” said interventional cardiologist Tanvir Bajwa, MD, Advocate Aurora Research Institute’s principal investigator for the study. “A faulty mitral valve has traditionally been replaced or repaired in surgery, but about half of all people with severe mitral regurgitation are poor candidates for open heart surgery because of underlying health issues. This makes a minimally invasive, catheter-based procedure an appealing option.”
In a TMVR procedure, a team of cardiologists and cardiac surgeons inserts an artificial replacement mitral valve via a tube called a catheter through a small incision in the femoral vein in the leg. The doctors then carefully thread the catheter, guided by imaging technology, through the body’s chain of blood vessels until it reaches the correct location in the heart, at which point the replacement valve is implanted and the catheter is removed.
“Catheter-based treatments are often used to replace other heart valves and for repair of the mitral valve, but there are currently no U.S. Food and Drug Administration-approved catheter-based technologies that replace the entire mitral valve,” Dr. Bajwa said.
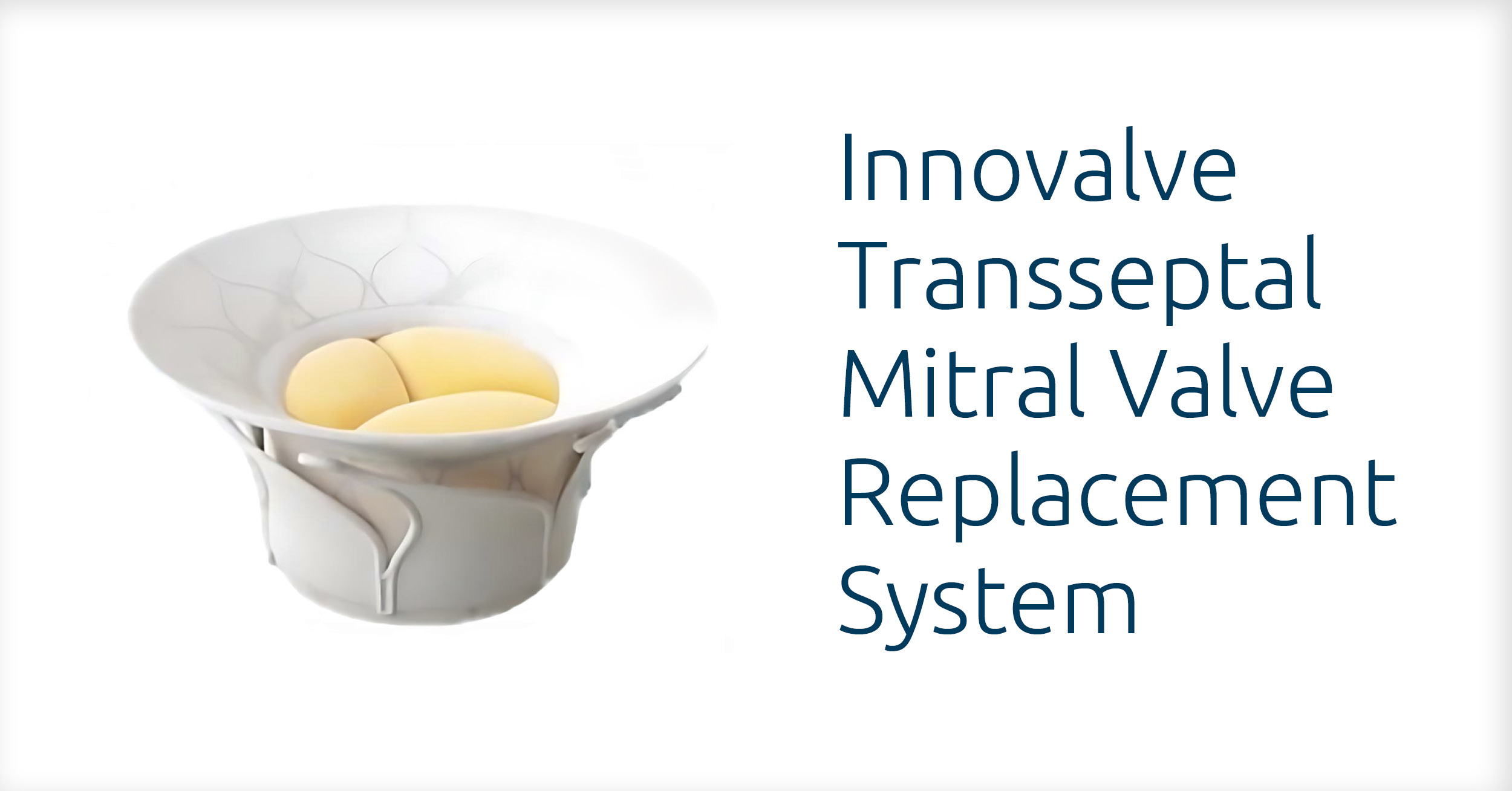
The investigational device in the TWIST study is called the Innovalve Transseptal Mitral Valve Replacement System. This is the first research study to evaluate the device in humans.
“This kind of research is considered an early feasibility study, in which researchers evaluate innovative devices or procedures in a small number of people to gauge basic safety and performance,” said Laura Wrona, MSN, Director of the Research Institute’s Center for Excellence in Cardiovascular Research. “Advocate Aurora Research Institute investigators and Aurora St. Luke’s Medical Center cardiologists have been leaders in the field of minimally invasive heart valve procedures, beginning with their early participation in transcatheter aortic valve replacement, or TAVR, clinical trials.”
To date, Aurora St. Luke’s cardiologists have performed more than 3,000 minimally invasive valve replacement and repair procedures.
The TWIST trial is sponsored by Innovalve Bio Medical Ltd., manufacturer of the study device.
To learn more about Advocate Aurora’s research, visit aah.org/research.